This document is applicable to biological evaluation of all types of medical devices including active, non-active, implantable and non-implantable medical
ISO 55001:2014 specifies requirements for an asset management system within the context of the organization. ISO 55001:2014 can be applied to all types of assets…
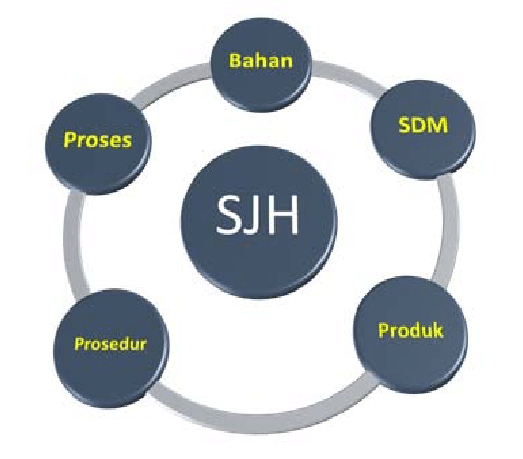
The system has considered food safety, quality and cleanliness at each level of production. It provides a systematic approach to ensure and preserve halal integrity…
Current good manufacturing practices are those conforming to the guidelines recommended by relevant agencies. Those agencies control the authorization and licensing of the manufacture…
Standar ISO 21001: 2018 adalah standar internasional yang menetapkan persyaratan untuk sistem manajemen mutu bagi organisasi pendidikan dalam mencapai tujuan dan menjalankan fungsi utamanya, yaitu memberikan…
ISO 14971 addresses risk management and is the international standard designed for the medical device industry. This standard defines the best practices throughout the entire…
ISO 31000:2018 menekankan tujuan manajemen risiko, yaitu menciptakan dan melindungi nilai. Tujuan itu diwujud- kan dengan (1) meningkatkan kinerja, (2) mendorong inovasi, dan (3) mendukung…
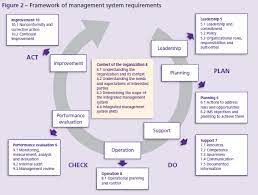
PAS 99:2012 adalah standar pertama di dunia sebagai sistem manajemen terintegrasi. Mengintegrasikan sistem manajemen PAS 99:2012 memberikan kesempatan organisasi untuk mendapatkan lebih banyak manfaat…
Since 2007 and until its latest version update in 2022, this standard sets the requirements for quality management, technical processes, and competence in medical laboratories….
QSI Certification – ISO/TS 16949 is the ISO series for automotive management systems, the basic framework of this system still uses ISO 9001 with…
HACCP is a systematic, science-based method that identifies specific hazard risks and control measures to ensure the safety of manufactured food products. Focusing on…
The EU’s Medical Device Regulation (MDR) was officially published on 5 May 2017 and came into force on 25 May 2017. The MDR replaced…
- « Previous
- 1
- 2
- 3
- Next »